The 30-year-old PPE Directive 89/686 has been replaced by PPE Regulation 2016/425. The following FAQ contains more information on the impacts and rollout of the change.
Why the change?
The directive is nearly 30 years old and the personal protective equipment market has undergone significant change since its inception. It was therefore necessary to update the text of the directive to adapt to changes in the market. This change allows for better definition of the roles and responsibilities for everyone involved in the market: manufacturers, importers, distributors and notified bodies.
What is the difference between a directive and a regulation?
A regulation is a community law that applies to all member states in the Union. It is directly applicable as written. A directive must be implemented by the member state itself. In other words, it is transcribed. Which means that the state is free to choose the means by which it will implement the law. It must implement the directive by a certain deadline.
What are the major changes?
- A more precise definition of roles and responsibilities for the different economic players to adapt to changes in the market.
- Clearer definitions of the different PPE categories (I, II and III). In addition, the validity of EU type certificates is limited to 5 years: the product is thus re-evaluated and re-tested if needed every 5 years, depending on changes to standards.
- New requirements for marking and for information to be provided in the Instructions for Use (see following paragraph).
How does a product display compliance with the new regulation?
The regulation has new requirements for marking and information supplied to the user:
- Marking
- The manufacturer's address must appear on the product, or on the instructions or packaging if space is lacking
- For products subject to aging (textiles/helmets), the date of manufacture (month/year) must be attached to the product and packaging. Note: the Petzl serial number also includes a date of manufacture. There may be a few months' difference between the two dates. The relevant date for your PPE inspection is the month/year date of manufacture. This difference is due to our internal manufacturing process.
- Instructions for use
- Must describe the hazard that the product is designed to protect against.
- Must refer to PPE Regulation 2016/425.
- Must display the URL of website where the declaration of conformity can be found.

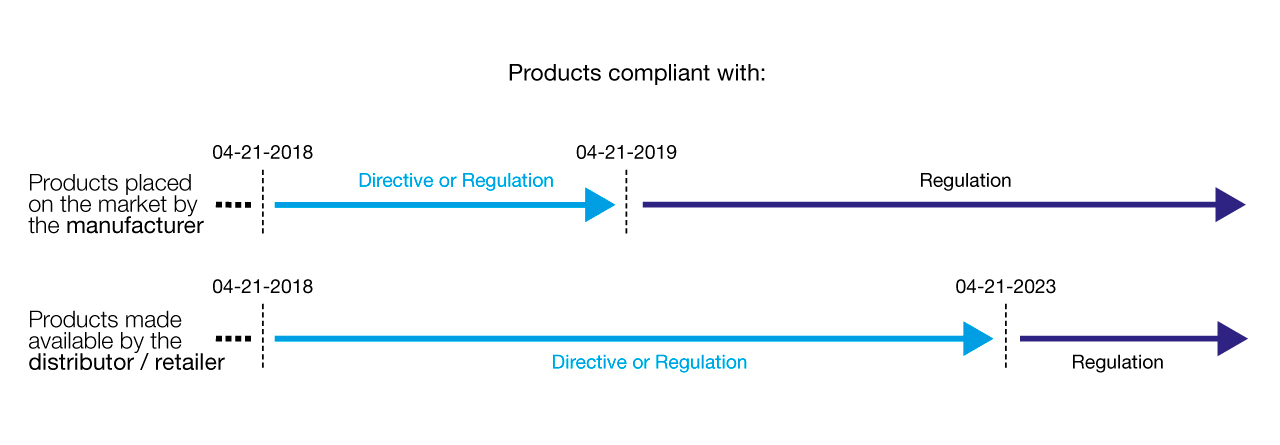
What is the implementation schedule?
- Implementation began on April 21, 2018:
Date after which it is possible to claim compliance with the regulation. - Between April 21, 2018 and April 21, 2019:
Transition period during which it is possible to sell products that are compliant with the directive or regulation. - Beyond April 21, 2019:
Our distributors and resellers may sell their remaining stock of directive-compliant products until April 21, 2023.
Schedule for implementation of the regulation:
Can I continue to use a product that complies with the directive?
As a user, you can continue to use a directive-compliant product throughout the life of the product. For more information, the entire regulation may be found here.



